Analyse HeLa fluorescence siRNA screen

OverviewQuestions:Objectives:
How do I analyze a HeLa fluorescence siRNA screen?
How do I segment cell nuclei?
How do I extract features from segmentations?
How do I filter segmentations by morphological features?
How do I apply a feature extraction workflow to a screen?
How do I visualize feature extraction results?
Requirements:
How to segment cell nuclei in Galaxy.
How to extract features from segmentations in Galaxy.
How to filter segmentations by morphological features in Galaxy.
How to extract features from an imaging screen in Galaxy.
How to analyse extracted features from an imaging screen in Galaxy.
- Introduction to Galaxy Analyses
- Imaging
- Introduction to image analysis using Galaxy: tutorial hands-on
Time estimation: 1 hourLevel: Intermediate IntermediateSupporting Materials:Last modification: Oct 18, 2022
Introduction
This tutorial shows how to segment and extract features from cell nuclei Galaxy for image analysis. As example use case, this tutorial shows you how to compare the phenotypes of PLK1 threated cells in comparison to a control. The data used in this tutorial is available at Zenodo.
RNA interference (RNAi) is used in the example use case for silencing genes by way of mRNA degradation. Gene knockdown by this method is achieved by introducing small double-stranded interfering RNAs (siRNA) into the cytoplasm. Small interfering RNAs can originate from inside the cell or can be exogenously introduced into the cell. Once introduced into the cell, exogenous siRNAs are processed by the RNA-induced silencing complex (RISC).The siRNA is complementary to the target mRNA to be silenced, and the RISC uses the siRNA as a template for locating the target mRNA. After the RISC localizes to the target mRNA, the RNA is cleaved by a ribonuclease. RNAi is widely used as a laboratory technique for genetic functional analysis. RNAi in organisms such as C. elegans and Drosophila melanogaster provides a quick and inexpensive means of investigating gene function. Insights gained from experimental RNAi use may be useful in identifying potential therapeutic targets, drug development, or other applications. RNA interference is a very useful research tool, allowing investigators to carry out large genetic screens in an effort to identify targets for further research related to a particular pathway, drug, or phenotype.
The example used in this tutorial deals with PLK1 knocked down cells. PLK1 is an early trigger for G2/M transition. PLK1 supports the functional maturation of the centrosome in late G2/early prophase and establishment of the bipolar spindle. PLK1 is being studied as a target for cancer drugs. Many colon and lung cancers are caused by K-RAS mutations. These cancers are dependent on PLK1.
AgendaIn this tutorial, we will deal with:
Getting data
The dataset required for this tutorial contains a screen of DAPI stained HeLa nuclei (more information). We will use a sample image from this dataset for training basic image processing skills in Galaxy.
Hands-on: Data upload
If you are logged in, create a new history for this tutorial
Click the new-history icon at the top of the history panel.
If the new-history is missing:
- Click on the galaxy-gear icon (History options) on the top of the history panel
- Select the option Create New from the menu
- Import galaxy-upload the following dataset from Zenodo or from the data library (ask your instructor).
- Important: Choose the type of data as
zip.https://zenodo.org/record/3362976/files/B2.zip
- Copy the link location
Open the Galaxy Upload Manager (galaxy-upload on the top-right of the tool panel)
- Select Paste/Fetch Data
Paste the link into the text field
Press Start
- Close the window
As an alternative to uploading the data from a URL or your computer, the files may also have been made available from a shared data library:
- Go into Shared data (top panel) then Data libraries
- Navigate to the correct folder as indicated by your instructor
- Select the desired files
- Click on the To History button near the top and select as Datasets from the dropdown menu
- In the pop-up window, select the history you want to import the files to (or create a new one)
- Click on Import
- Unzip file tool with the following parameters:
- param-file “input_file”:
Zippedinput file- “Extract single file”:
Single file- “Filepath”:
B2--W00026--P00001--Z00000--T00000--dapi.tifRename galaxy-pencil the dataset to
testinput.tif
- Click on the galaxy-pencil pencil icon for the dataset to edit its attributes
- In the central panel, change the Name field
- Click the Save button
- Unzip file tool with the following parameters:
- param-file “input_file”:
Zippedinput file- “Extract single file”:
All filesRename galaxy-pencil the resulting collection to
control
- Click on the collection
- Click on the name of the collection at the top
- Change the name
- Press Enter
- Import galaxy-upload the following dataset from Zenodo or from the data library (ask your instructor).
- Important: Choose the type of data as
zip.https://zenodo.org/record/3362976/files/B3.zip
- Copy the link location
Open the Galaxy Upload Manager (galaxy-upload on the top-right of the tool panel)
- Select Paste/Fetch Data
Paste the link into the text field
Press Start
- Close the window
As an alternative to uploading the data from a URL or your computer, the files may also have been made available from a shared data library:
- Go into Shared data (top panel) then Data libraries
- Navigate to the correct folder as indicated by your instructor
- Select the desired files
- Click on the To History button near the top and select as Datasets from the dropdown menu
- In the pop-up window, select the history you want to import the files to (or create a new one)
- Click on Import
- Unzip tool to extract the zipped screen:
- param-file “input_file”:
Zippedinput file- “Extract single file”:
All files- Rename galaxy-pencil the collection to
PLK1- Upload galaxy-upload the following segmentation filter rules as a new pasted file (format:
tabular):area eccentricity min 500 0. max 100000 0.5
- Open the Galaxy Upload Manager
- Select Paste/Fetch Data
Paste the file contents into the text field
Change Type from “Auto-detect” to
tabular- Press Start and Close the window
Rename galaxy-pencil dataset to
rules
- Click on the galaxy-pencil pencil icon for the dataset to edit its attributes
- In the central panel, change the Name field
- Click the Save button
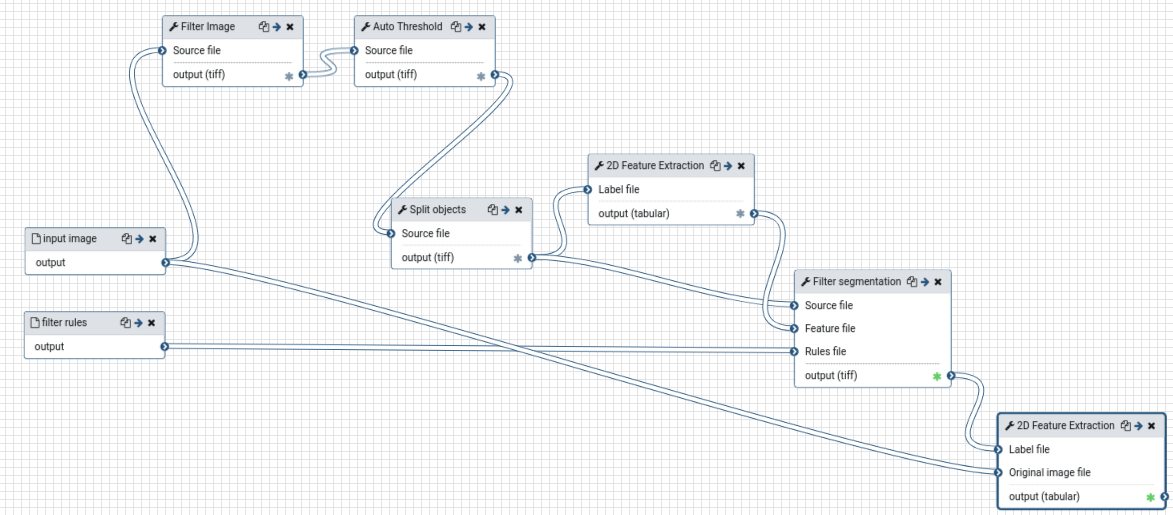
Create feature extraction workflow
First, we will create and test a workflow which extracts mean DAPI intensity, area, and major axis length of cell nuclei from an image.
Hands-on: Create feature extraction workflow
- Filter Image tool with the following parameters to smooth the image:
- “Image type”:
Gaussian Blur- “Radius/Sigma”:
3- param-file “Source file”:
testinput.tiffile- Auto Threshold tool with the following parameters to segment the image:
- param-file “Source file”: output of Filter image tool
- “Threshold Algorithm”:
Otsu- “Dark Background”:
Yes- Split objects tool with the following parameters to split touching objects:
- param-file “Source file”: output of Auto Threshold tool
- “Minimum distance between two objects.”:
20- 2D Feature Extraction tool with the following parameters to extract features from the segmented objects:
- param-file “Label file”: output of Split objects tool
- “Use original image to compute additional features.”:
No original image- “Select features to compute”:
Select features- “Available features”:
- param-check
Add label id of label image- param-check
Area- param-check
Eccentricity- param-check
Major Axis Length- Filter segmentation tool with the following parameters to filter the label map from 3. with the extracted features and a set of rules:
- param-file “Source file”: output of Split objects tool
- param-file “Feature file”: output of 2D Feature Extraction tool
- param-file “Rules file”: rules file
- 2D Feature Extraction tool with the following parameters to extract features the final readout from the segmented objects:
- param-file “Label file”: output of Filter segmentation tool
- “Use original image to compute additional features.”:
Use original image- param-file “Original image file”:
testinput.tiffile- “Select features to compute”:
Select features- “Available features”:
- param-check
Mean Intensity- param-check
Area- param-check
Major Axis Length- Now we can extract the workflow for batch processing
- Name it “feature_extraction”.
Clean up your history: remove any failed (red) jobs from your history by clicking on the galaxy-cross button.
This will make the creation of the workflow easier.
Click on galaxy-gear (History options) at the top of your history panel and select Extract workflow.
The central panel will show the content of the history in reverse order (oldest on top), and you will be able to choose which steps to include in the workflow.
Replace the Workflow name to something more descriptive.
Rename each workflow input in the boxes at the top of the second column.
If there are any steps that shouldn’t be included in the workflow, you can uncheck them in the first column of boxes.
Click on the Create Workflow button near the top.
You will get a message that the workflow was created.
- Edit the workflow you just created
- Name the inputs
input imageandfilter rules.- Mark the results of steps 5 and 6 as outputs (by clicking on the asterisk next to the output name).
The resulting workflow should look something like this:
Apply workflow to screen
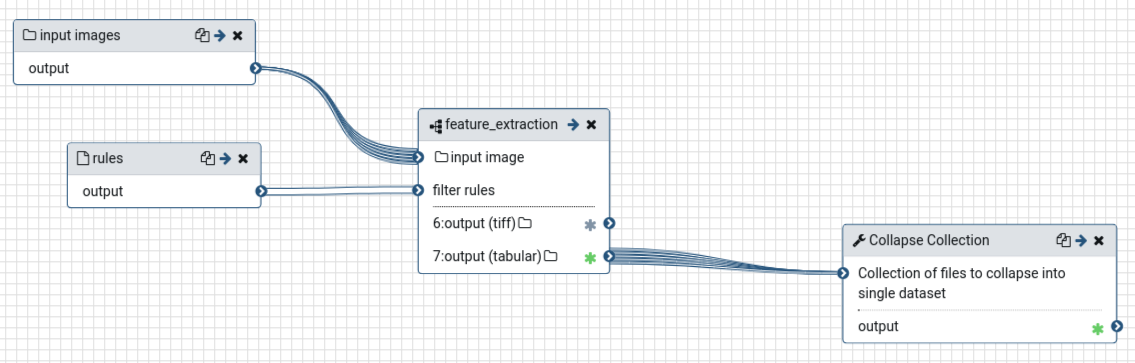
Now we want to apply our extracted workflow to original data and merge the results. For this purpose, we create a workflow which uses the previously created workflow as subworkflow.
Hands-on: Create screen analysis workflow
Create a new workflow in the workflow editor.
- Click Workflow on the top bar
- Click the new workflow galaxy-wf-new button
- Give it a clear and memorable name
- Clicking Save will take you directly into the workflow editor for that workflow
- Need more help? Please see the How to make a workflow subsection here
- Add a Input dataset collection node and name it
input images- Add a Input dataset node and name it
rules- Add the feature_extraction workflow as node.
- param-file “input image”:
input imagesoutput of Input dataset collection tool- param-file “filter rules”:
rulesoutput of Input dataset tool- Add a Collapse Collection tool node.
- param-file “Collection of files to collapse into single dataset”: output of feature_extraction workflow
- “Keep one header line”:
Yes- “Append File name”:
No- Mark the tool output as workflow output
- Save your workflow and name it
analyze_screen
The resulting workflow should look something like this:
Hands-on: Run screen analysis workflow
Run the screen analysis workflow workflow on the
controlscreen and therulesfile
- Click on Workflow on the top menu bar of Galaxy. You will see a list of all your workflows.
- Click on the workflow-run (Run workflow) button next to your workflow
- Configure the workflow as needed
- Click the Run Workflow button at the top-right of the screen
- You may have to refresh your history to see the queued jobs
Run the screen analysis workflow workflow on the
PLK1screen and therulesfile
Plot feature extraction results
Finally, we want to plot the results for better interpretation.
Hands-on: Plot feature extraction results
- Click on the
Visualize this datagalaxy-barchart icon of the Collapse Collection tool results.- Run
Box plotwith the following parameters:
- “Provide a title”:
Screen features- “X-Axis label”:
- “Y-Axis label”:
- “1: Data series”:
- “Provide a label”:
Mean intensity- “Observations”:
Column 1- “2: Data series”:
- “Provide a label”:
Area- “Observations”:
Column 2- “3: Data series”:
- “Provide a label”:
Major axis length- “Observations”:
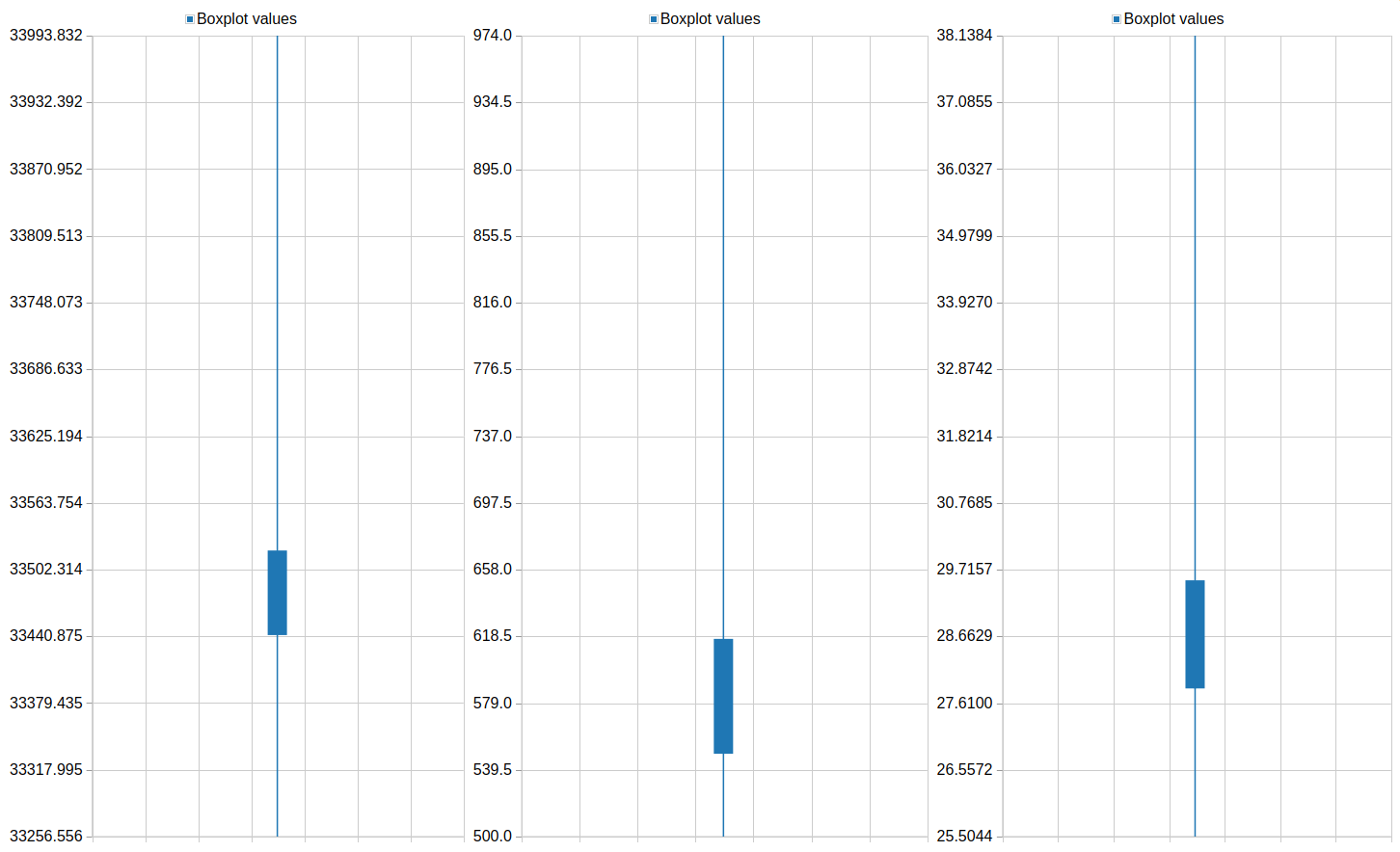
Column 3QuestionPlot the feature distribution of PLK1 and control. What differences do you observe between the screens?
The phenotype of PLK1 threated cells show a higher mean intensity and a shorter major axis in comparison to the control.
One of the resulting plots should look something like this:
Conclusion
In this exercise you imported images into Galaxy, segmented cell nuclei, filtered segmentations by morphological features, extracted features from segmentations, scaled your workflow to a whole screen, and plotted the feature extraction results using Galaxy.
Key points
Galaxy workflows can be used to scale image analysis pipelines to whole screens.
Segmented objects can be filtered using the Filter segmentation tool.
Galaxy charts can be used to compare features extracted from screens showing cells with different treatments.
Frequently Asked Questions
Have questions about this tutorial? Check out the tutorial FAQ page or the FAQ page for the Imaging topic to see if your question is listed there. If not, please ask your question on the GTN Gitter Channel or the Galaxy Help ForumUseful literature
Further information, including links to documentation and original publications, regarding the tools, analysis techniques and the interpretation of results described in this tutorial can be found here.
Feedback
Did you use this material as an instructor? Feel free to give us feedback on how it went.
Did you use this material as a learner or student? Click the form below to leave feedback.
Citing this Tutorial
- Thomas Wollmann, 2022 Analyse HeLa fluorescence siRNA screen (Galaxy Training Materials). https://training.galaxyproject.org/training-material/topics/imaging/tutorials/hela-screen-analysis/tutorial.html Online; accessed TODAY
- Batut et al., 2018 Community-Driven Data Analysis Training for Biology Cell Systems 10.1016/j.cels.2018.05.012
Congratulations on successfully completing this tutorial!@misc{imaging-hela-screen-analysis, author = "Thomas Wollmann", title = "Analyse HeLa fluorescence siRNA screen (Galaxy Training Materials)", year = "2022", month = "10", day = "18" url = "\url{https://training.galaxyproject.org/training-material/topics/imaging/tutorials/hela-screen-analysis/tutorial.html}", note = "[Online; accessed TODAY]" } @article{Batut_2018, doi = {10.1016/j.cels.2018.05.012}, url = {https://doi.org/10.1016%2Fj.cels.2018.05.012}, year = 2018, month = {jun}, publisher = {Elsevier {BV}}, volume = {6}, number = {6}, pages = {752--758.e1}, author = {B{\'{e}}r{\'{e}}nice Batut and Saskia Hiltemann and Andrea Bagnacani and Dannon Baker and Vivek Bhardwaj and Clemens Blank and Anthony Bretaudeau and Loraine Brillet-Gu{\'{e}}guen and Martin {\v{C}}ech and John Chilton and Dave Clements and Olivia Doppelt-Azeroual and Anika Erxleben and Mallory Ann Freeberg and Simon Gladman and Youri Hoogstrate and Hans-Rudolf Hotz and Torsten Houwaart and Pratik Jagtap and Delphine Larivi{\`{e}}re and Gildas Le Corguill{\'{e}} and Thomas Manke and Fabien Mareuil and Fidel Ram{\'{\i}}rez and Devon Ryan and Florian Christoph Sigloch and Nicola Soranzo and Joachim Wolff and Pavankumar Videm and Markus Wolfien and Aisanjiang Wubuli and Dilmurat Yusuf and James Taylor and Rolf Backofen and Anton Nekrutenko and Björn Grüning}, title = {Community-Driven Data Analysis Training for Biology}, journal = {Cell Systems} }
Do you want to extend your knowledge? Follow one of our recommended follow-up trainings:
- Statistics and machine learning
- Basics of machine learning: tutorial hands-on
 Questions:
Questions: